Genetics and Infertility: A Complex Relationship
Genetics play a role in up to 10% of couples experiencing infertility or recurrent pregnancy loss.
Genetic testing is a medical tool used to identify changes in chromosomes, genes, or proteins. According to 23andMe, the results of a genetic test can confirm or rule out a suspected condition or help determine a person’s risk of developing or passing on a genetic disorder.
In the field of reproductive medicine, genetics can present both opportunities and challenges. Sometimes they offer clarity; other times, they raise new questions. In many ways, genetics can be either a friend or a foe—so how much can we truly rely on them?
Certain genetic and chromosomal abnormalities are clearly linked to repeated in vitro fertilization (IVF) failures or recurrent miscarriages. Today, DNA analysis has become a powerful diagnostic tool, capable of detecting conditions that once went unnoticed until later in pregnancy—or even after a miscarriage had occurred. It is now undeniable that genetic testing is not only here to stay, but is continuously evolving to offer deeper insights.
Through genetic testing, we can assess DNA integrity and identify potential diseases, mutations, or chromosomal alterations. This information is invaluable to prospective parents and their medical teams in selecting the most appropriate treatment pathway, especially when there is a known medical or family history.
Humans have a karyotype consisting of 23 pairs of chromosomes, for a total of 46 chromosomes in each cell. Of these, 22 pairs are called autosomes, while the remaining pair consists of sex chromosomes (XX or XY).
Any change in the number of chromosomes can affect growth, development, and the functioning of body systems. These alterations may occur during the formation of reproductive cells (egg and sperm), in early fetal development, or even after birth in somatic cells. A gain or loss of chromosomes—referred to as aneuploidy—is typically the result of a sporadic error in cell division known as nondisjunction.
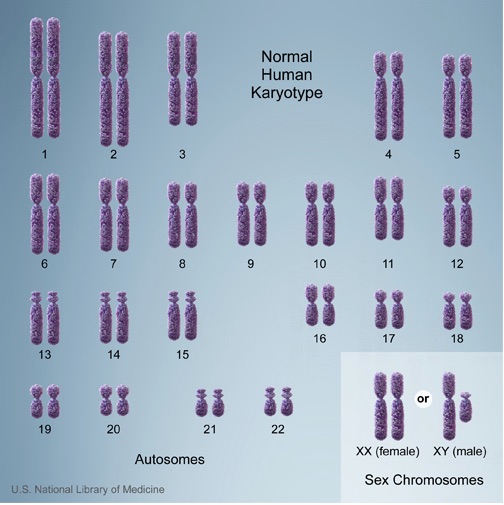
A common form of aneuploidy is trisomy, which occurs when there is an extra chromosome present in the cells. A well-known example is Down syndrome, characterized by an additional copy of chromosome 21 (trisomy 21). In contrast, monosomy refers to the loss of a chromosome. An example is Turner syndrome, where one of the X chromosomes is missing in females, resulting in a 45,X karyotype.
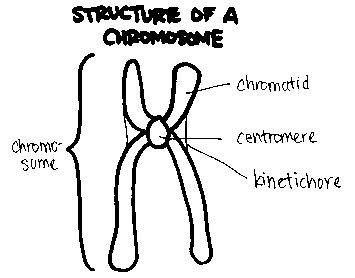
Each chromosome is structurally divided into three main parts:
- The p arm, which is the short (top) section
- The centromere, the central region that connects the arms and plays a critical role during cell division
- The q arm, which is the long (bottom) section
This structural organization is important in identifying chromosomal abnormalities and understanding how they affect genetic function.
Genetic Factors in Male and Female Infertility
In males, certain infertility conditions are linked to genetic risks. These include non-obstructive azoospermia, oligospermia, and congenital absence of the vas deferens (either bilateral or unilateral). Such conditions are often associated with chromosomal abnormalities or microdeletions of the Y chromosome, which can affect sperm production and transport.
In females, age remains the most significant factor influencing fertility. As a woman ages, both the quantity and quality of her eggs decline. While a healthy 30-year-old woman has about a 20% chance of conceiving per cycle, a woman at age 40 has approximately a 5% chance. This decline is also reflected in an increased risk of chromosomal abnormalities in embryos.
Genetic testing can be valuable at any stage of life—from the embryonic stage to advanced age—providing crucial insights for diagnosis, treatment planning, and reproductive decision-making.
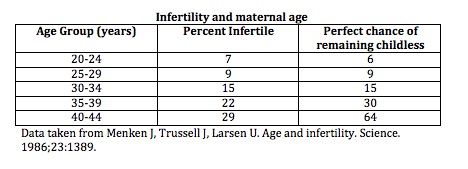
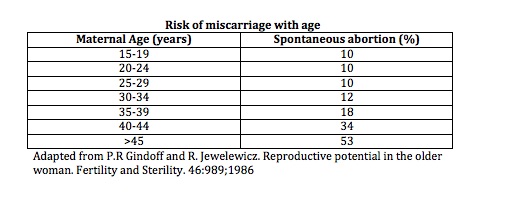
Genetic Screening in Women Over 40 and Those at Risk
As maternal age increases, so does the risk of fetal chromosomal abnormalities. Women over the age of 40 are especially encouraged to consider genetic testing in conjunction with IVF or ICSI procedures to improve outcomes and reduce the risk of complications.
It is advisable to offer preimplantation genetic testing for aneuploidy (PGT-A) or preimplantation genetic diagnosis (PGD), as well as prenatal screening and diagnostic tests for fetal aneuploidy, particularly in women over 40 or those with a personal or family history of chromosomal disorders.
Prenatal screening includes non-invasive options such as:
- Maternal serum screening
- Nuchal translucency ultrasound
- Non-invasive prenatal testing (NIPT), which analyzes fetal DNA in maternal blood
Invasive diagnostic procedures include:
- Chorionic villus sampling (CVS), typically performed at 10–12 weeks of gestation
- Amniocentesis, performed at 15 weeks or later
These tests are also valuable for women with recurrent miscarriage. Research has shown that approximately 60% of pregnancy losses in couples with 2–4 miscarriages are due to chromosomal abnormalities (Ogasawara et al., 2000), and up to 76% of embryos from patients with more than 3 miscarriages are chromosomally abnormal (Pellicer et al., 1999).
Another genetic condition to be aware of in women is primary ovarian insufficiency (POI). It is typically diagnosed by elevated FSH levels in women under 35, though it usually presents between ages 40 and 50, coinciding with early menopause. When POI occurs prematurely, it has been associated with mutations in the FMR1 gene located on the X chromosome.
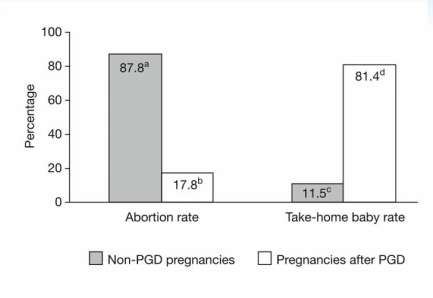
The following data, sourced from the American Society for Reproductive Medicine (ASRM), illustrates the reduction in pregnancy loss rates observed after the use of preimplantation genetic diagnosis (PGD).

This following data shows the percentage of abortion rate versus take-home babies with and without preimplantation genetic testing.
Conclusion
Genetic testing offers significant value in the prevention, diagnosis, and management of chromosomal anomalies and genetic alterations. It allows us to identify potential risks early on, often before any symptoms or issues arise. There is no need to wait for a problem to manifest when proactive screening can provide vital information for clinical decision-making.
However, access to genetic testing remains limited for some patients due to high costs and the logistical challenges involved—such as the need to freeze embryos while awaiting results in the case of preimplantation genetic testing (PGT).
It is crucial for healthcare professionals to remain informed and current with the latest advances and technologies. What was once undetectable is now within reach, thanks to progress in molecular diagnostics and reproductive medicine.
That said, it is important to note that a negative genetic test result does not completely rule out the possibility of an underlying, undetected genetic cause. Continued research and technological advancement are essential for broadening our understanding and diagnostic capabilities.
Acknowledgments
Gratitude to the following sources for their contributions and references:
Reproductive Medicine Associates of Connecticut
Genetics Home Reference
American Society for Reproductive Medicine (ASRM)
23andMe